Medical PCB Assembly for Healthcare Devices
Dependable medical component manufacturing where precision, repeatability, and trust are non-negotiable.
When Lives Depend on Electronics, Precision Comes First
When you source medical PCB assembly, you’re not just ordering a component — you’re protecting a device, a timeline, and the people who will ultimately rely on it.
ETEMCO is ISO 9001:2015 certified and built for teams who don’t gamble on uncertainty. We provide full turn-key PCB manufacturing for medical OEMs across PA, NJ, DE, and OH, giving you verified precision, clean documentation, and confidence that every build is controlled from day one.
Common Medical Applications We Deliver
ETEMCO provides PCB assemblies for a wide range of regulated healthcare electronics, including:
- Diagnostic & imaging systems
- Patient monitoring devices
- Surgical & clinical equipment
- Portable & wearable health tech
- Medical equipment PCB
Engineering Support & Early Design Collaboration
From Sourcing Through Final Inspection
In the early stages of a design, what most teams need isn’t more speed — it’s clarity. You need confidence that the board can be manufactured reliably, that components will remain available, and that the design won’t fall apart during validation.
Our engineering review supports custom PCB design for medical devices and medical PCBA, providing manufacturability confidence before tooling or sourcing decisions become permanent, especially in healthcare electronic manufacturing services where validation and reliability matter.
The result is reduced risk, cleaner handoffs, and the assurance that you remain in control before costly changes show up downstream.

Traceability, Validation & ISO-Aligned Processes
In medical manufacturing, you can’t just hope a supplier ‘got it right’, you need proof. When you choose ETEMCO, traceability is built into how your boards are produced, reviewed, and documented.
Our ISO 9001:2015–aligned process includes:
- Serialized lot and component tracking
- Recorded inspections and process checkpoints
- Supplier accountability and material verification
- Documented audit trails for every build
Instead of chasing missing documentation later, you get clean validation and defensible evidence from the start. You stay confident and supported through audits, not exposed to them.
Full Turn-Key PCB Manufacturing — From Sourcing Through Final Inspection
In medical manufacturing, every extra supplier is another handoff to monitor and another place where documentation can break down. A turn-key process gives you one accountable partner, one audit trail, and no gaps between sourcing, assembly, and validation.
ETEMCO keeps everything under one roof, so when something changes: an ECO, component shift, or tolerance update, it’s handled quickly and cleanly. You stay in control without having to chase multiple vendors.
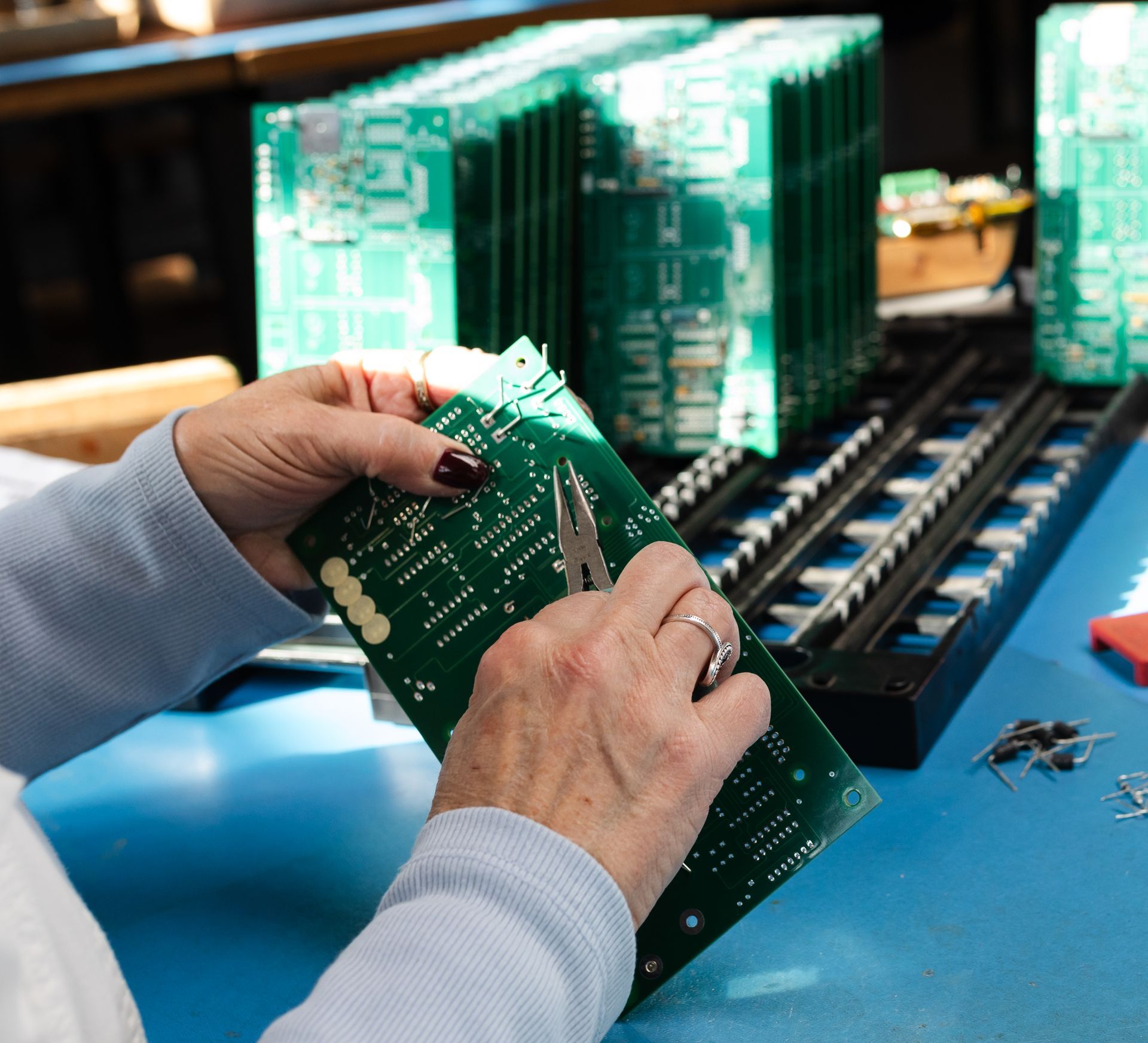
Our Core Electronics Capabilities
ETEMCO’s core processes are built for medical reliability; stable, repeatable, and controlled from start to finish.

Surface Mount PCB Assembly
ETEMCO has high-speed surface mount pick and place equipment that allows us to offer our customers cost effective and high-quality production services. Our flexible equipment allows efficient production of low to high volumes, and simple to highly complex assemblies.

Thru-Hole Electronics Assembly
Skilled technicians and selective solder equipment ensure clean, durable joints for components requiring strength. Your boards gain long-term mechanical reliability, especially important for devices exposed to movement or handling.

Selective Solder Assembly
Automated selective solder delivers controlled, predictable results for mixed-technology assemblies.
Eliminates the variability of manual soldering and protects product consistency during audits.
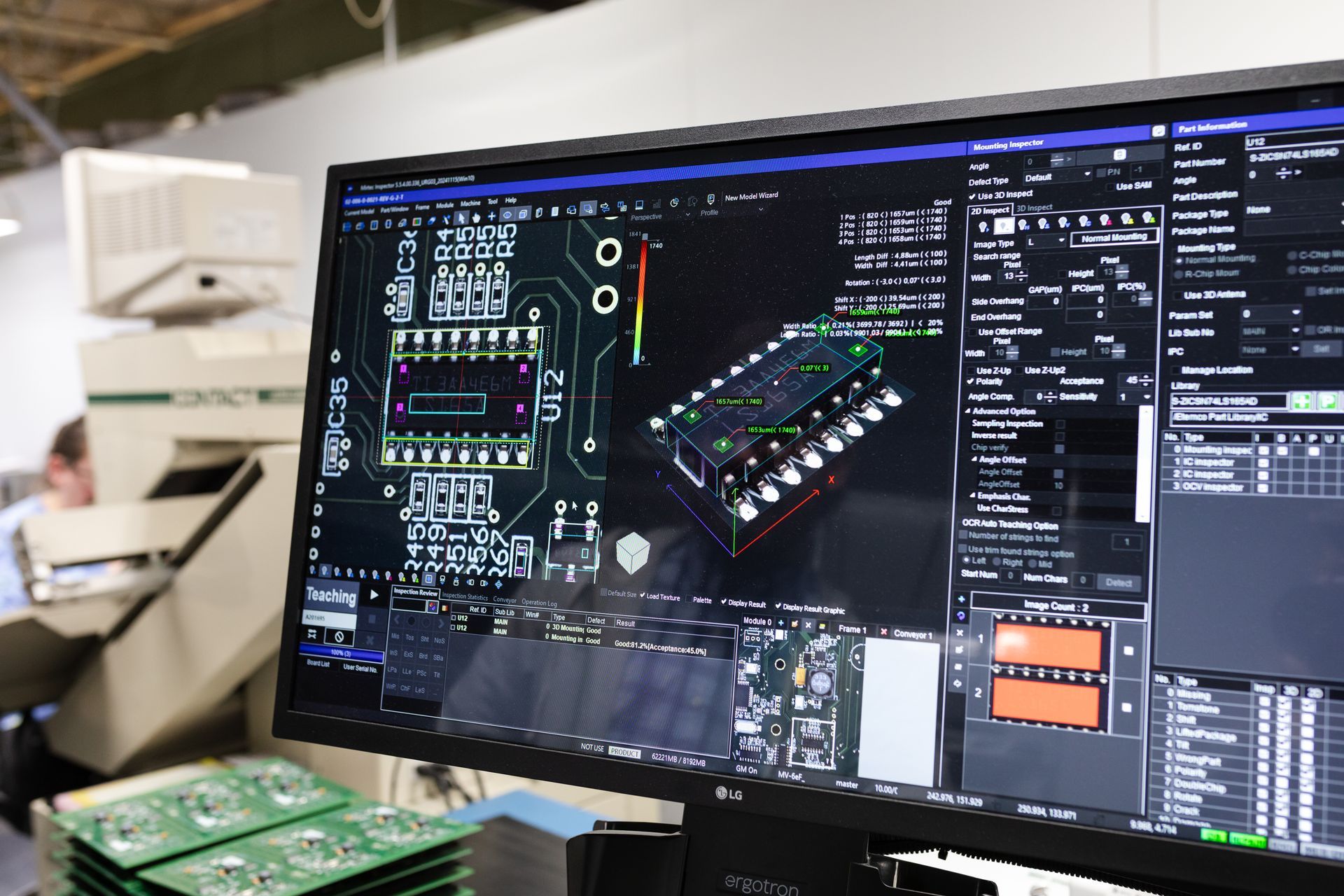
Automated Optical Inspection (AOI)
Every board is scanned for placement, polarity, solder integrity, and alignment. Defects are caught early — reducing rework and protecting your validation timeline.

Conformal Coating & RTV Dispensing
Controlled application of coatings and RTV protects sensitive circuitry from moisture, chemicals, and vibration.
Your devices maintain performance in challenging clinical environments without coating-related failures.

PDR Infrared Rework
Infrared rework systems remove and replace components without overheating or damaging the PCB. You recover boards safely, avoid scrapping expensive assemblies, and maintain full documentation control.
Design for Manufacturing (DFM/DFA)
Early manufacturability and component availability reviews prevent issues before tooling begins.
Your engineering team avoids costly redesigns and stays on schedule through validation and production.
Why Partner With ETEMCO as Your Healthcare PCB Manufacturer?
For more than 70 years, ETEMCO has built medical PCB assemblies here in the region — not offshore — which means your boards stay close, communication stays fast, and your timeline isn’t held up by distance or vendor turnover.
Our workforce is highly trained (IPC-600 and beyond), but healthcare and medical device companies choose ETEMCO because we offer something bigger than capability. With us, you get consistency, responsiveness, and a manufacturing partner you can actually reach.
The Regions We Proudly Serve
Pennsylvania • New Jersey • Delaware • Ohio • Nationwide
Additional Industries We Support
While medical and healthcare manufacturing is a core focus, ETEMCO also supports a wide range of regulated and high-reliability industries including:
- Telecommunications & Networking
- Industrial Automation & Controls
- Military & Law Enforcement
- Transportation & Mobility
- Security & Access Control
Medical PCB Assembly Questions Procurement Teams Ask Most
What is your typical lead time for medical PCB assembly?
Lead times vary by complexity, but most medical builds begin within 2–4 weeks. For prototypes, we can often begin sooner depending on documentation readiness.
Do you support low-volume or prototype medical PCB production?
low- to medium-volumeYes. ETEMCO supports prototyping and low-to-medium-volume medical PCB assemblies, allowing engineering teams to validate early builds before scaling into higher production volumes.
How does ETEMCO handle traceability for medical devices?
Every build is serialized and fully documented, with component-level tracking, recorded inspections, and a controlled audit trail. This gives you defensible proof during validation and supplier reviews.
Are your medical PCB assemblies manufactured in the United States?
Yes. All medical PCB assemblies are built in Lancaster, PA by an experienced, IPC-trained workforce — not offshore labor. This shortens lead times, preserves documentation integrity, and gives you a partner you can actually reach.
Your Work Protects People. Our Precision Protects Your Work.
When reliability is non-negotiable, you need more than a vendor; you need a partner you can trust. ETEMCO delivers medical PCB assembly with the precision, documentation, and accountability your work depends on.
You stay confident, supported, and in control, knowing your build is in responsible hands from start to finish.